Natural Health Products (NHP)
Natural Health Products (NHP) are substances derived from natural sources, such as plants, minerals, and animals, used to maintain, promote, or restore good health. These products include vitamins, minerals, herbal remedies, homeopathic medicines, and more. NHP offers an alternative and complementary approach to traditional healthcare practices.
Who needs it?
If you are involved in the production, distribution, or importation of health products derived from natural sources, obtaining Natural Health Products (NHP) licensing is crucial. This license applies to various industries, including but not limited to manufacturers, retailers, wholesalers, herbalists, healthcare clinics, and pharmacies.

Importance
- Regulatory Compliance: Acquiring the Natural Health Products (NHP) license ensures that your business operates in accordance with regulations set by Health Canada, ensuring public safety and product quality.
- Consumer Trust: Holding the NHP license instills confidence in your customers, demonstrating your commitment to quality, safety, and providing reliable natural health products.
- Market Expansion: Having an NHP license enables you to tap into the growing market of consumers seeking natural and alternative health products. It opens up opportunities for new partnerships, increased sales, and reaching a wider customer base.
NHP Licensing Process
- Preparation and Documentation:
Gather all necessary documentation, including product formulation, specifications, labeling, and safety/effectiveness evidence. - Product Notification and Submission:
Depending on NHP classification, submit a product notification or a full submission to Health Canada, including required forms, fees, and supporting documents. - Review and Assessment:
Health Canada reviews submission for compliance with regulatory requirements, assessing safety, efficacy, quality, and labeling. - Decision and Approval:
Health Canada makes a decision on application; if approved, issues a Natural Product Number (NPN). - Compliance and Maintenance:
Maintain compliance with ongoing regulatory requirements, including Good Manufacturing Practices (GMP) and post-market notifications. - Post-Market Surveillance:
Health Canada conducts post-market surveillance to monitor NHP safety and quality, requiring cooperation from license holders for adverse event reporting.
Natural Health Product Registration Fees:
|
NHP category |
Fee amount ($ CAD) |
|
Class I application or amendment |
$2,761 |
|
Class II application or amendment |
$30,677 |
|
Class III application or amendment |
$7,209 |
|
Class III novel application |
$58,332 |
|
Class III novel safety and efficacy amendment |
$23,333 |
|
Class III novel quality amendment |
$8,750 |
|
Site License applications or amendments |
$4,784 |
|
Annual Site License - manufacturing - sterile dosage form |
$40,071 |
|
Annual Site License - manufacturing - non-sterile dosage form |
$20,035 |
|
Annual Site License - packaging |
$7,650 |
|
Annual Site License - labelling |
$6,921 |
|
NHP RTS (Right to Sell Fees) |
$542 |
|
Building outside Canada (each) |
$949 |
Application Fees
Proposed NHP fees and performance standards (existing standards included where applicable).
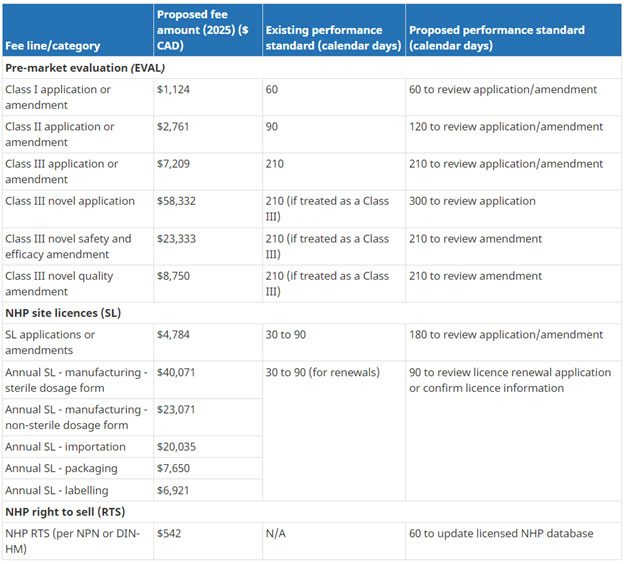
Note: Unit costs were adjusted to cover the time between when the costing data was collected/calculated and when the fees will be implemented: 3.4% for 2021-22 and 2% for each of 2022-23, 2023-24 and 2024-25.
Get in Touch with Your Licensing Expert
Find out what licensing solution would work best for your business.
For more information on our services and to help you secure your business, contact us below.

